Engineering Excellence in Projects and Facilities
Life Cycle Management
PRIME SOLUTIONS (PRIME) is an Engineering and Consulting Company with a holistic approach for Medical Facilities Life Cycle Management from Medical Projects development to Facility Management and Engineering Operations.
About us
Leading Innovation in Healthcare Facility Engineering
Mission Statement
PRIME SOLUTIONS mission is to enhance healthcare delivery through innovative engineering solutions aimed to improve and safeguard healthcare infrastructures throughout their entire life cycle. The company is dedicated to enhancing the integrity, safety and functionality of healthcare infrastructure throughout the facility life cycle with evidence-based project delivery, proactive facility management and biomedical engineering. Their focus is on optimizing both capital and operational costs, maximizing efficiency, and resource utilization for healthcare providers while preserving patient and staff safety.
Vision Statement
PRIME SOLUTIONS aims at becoming a leader in engineering solutions for the medical sector, recognized for excellence and innovation in healthcare infrastructure development and management. They aspire to empower healthcare organizations through their expertise in facility maintenance, biomedical engineering and project management, enhancing performance, safety, efficiency, and sustainability. This commitment aims to improve patient outcomes and contribute positively to community health.
- Commitment to Quality
Striving for excellence in all aspects of healthcare facilities, from project delivery to infrastructure operations - Innovation
Integrating activity-based design, new medical technologies, and operational models to enhance patient care and safety - Collaboration
Partnering with healthcare providers, architects, and engineers to enhance healthcare facilities throughout their lifecycles
Our services
Comprehensive Services For Every Phase of Your Project Journey
Our comprehensive project implementation services cover all project stages...A Holistic Facility Life Cycle Management
Our services cover the full lifecycle of facilities’ infrastructure...One Focal Point and Integrated Services:
Rather than having to coordinate with multiple internal and external stakeholders, we can represent the client as the focal point for all infrastructure and fixed assets maintenance activities streamlining the management of all internal maintenance activities and effectively coordinating with external service providers and contracted companies.
Operational efficiency and Quality of Service:
Our commitment to operational efficiency and quality service through streamlined processes that boost productivity.
Our preventive and proactive maintenance programs adhere to industry standards, with regular inspections to identify and address potential issues, ensuring safety and compliance in your facilities.
Value Engineering and Cost Saving:
With our extensive experience and benchmarking expertise, we deliver evidence-based solutions that identify effective strategies and explore all alternatives to enhance quality while reducing costs.
As operational costs represent a significant portion of healthcare facility expenses, we also collaborate with clients to implement energy-efficient solutions that promote sustainability and environmental responsibility.
Accreditation and Regulatory Compliance:
With our long standing expertise in the field, we master navigating the complex regulatory landscape of healthcare including JCI and healthcare facilities accreditation requirements, ensuring that facilities meet all necessary compliance requirements.
Operations Continuity and Technical Staff Turnover:
Rather than being concerned about potential technical staff turnover (engineers or technicians), we offer seamless operational continuity and professional technical staff for healthcare facilities through both on-site and support teams.
Risk Management and Emergency Preparedness:
We prioritize our clients’ safety by conducting thorough risk assessments and implementing a continuous monitoring system and risk mitigation strategies to identify and reduce potential hazards related to safety, security, and compliance.
Computerized Maintenance Management System (CMMS):
Using our CMMS system enhances operational efficiency by centralizing maintenance operations, automating workflows, and enabling data-driven decisions across healthcare infrastructure and biomedical assets. Seamless integration with client ERP systems ensures real-time data exchange, improved compliance, centralized asset management, and significant cost savings—ultimately boosting both our operational performance and client outcomes.
Data-Driven Decision Making:
Using customized KPIS, reports and Dashboards we focus on monitoring each client’s specific pain points.
This tailored approach allows us to gain a deep understanding of the unique challenges faced by each facility, enabling us to collaborate with the client to implement informed data –driven decision making and solutions.

Needs Assessment
Hospital design is a collaborative endeavor between a large number of stakeholders from different backgrounds, ranging from medical, nursing, administrative, financial, legal, biomedical and engineering to all technical engineering trades.
Before medical planning can start, and in order to translate the client’s vision into a unique healthcare project in alignment with the market needs and feasibility study outcome, we conduct a needs assessment through a series of key stakeholders workshops, key informant interviews, and data collection questionnaires that gather the client team and end-users feedback and define the medical facility infrastructure and medical technologies requirements, to ultimately provide an activity based design solution in line with the client’s vision.

The needs assessment covers mainly the overall facility intended operational models, end-users needs, medical and support departments workloads and peak periods, deployed medical technology features, and complexity levels. The needs assessment combined data will ultimately guide the medical planning process for specialized departments like the operating theater, CSSD, outpatient clinics, laboratory, and pharmacy, in addition to the definition and selection of technologies and systems needs like medical equipment, information technology integration, medical gas, RTLS, and NCS, along with their space needs and cost budgeting implications. In the absence of information, our multidisciplinary team can advise the client to make the proper assumptions based on the best practices, market needs, and applicable guidelines and requirements.
Our methodology for needs assessment merges the client and end-users’ requirements and vision with the adopted medical guidelines and standards into the needs assessment report that will define all future planning and design criteria.
The deliverable of this phase – Needs Assessment Report – is a narrative document that will constitute the basis of all the subsequent planning phase.

Functional Program
The Functional Program is a detailed description of the clinical, operational, and support services a healthcare facility must provide, translated into requirements for space, flow, staffing, equipment, and infrastructure.

It answers:
✅ What services will be offered?
✅ How will patients, staff, and materials move?
✅ What spaces are needed, and how should they relate?
✅ What systems (IT, HVAC, infection control) are required?
Our approach to functional program development integrates client preferences with national and international standards such as FGI, HBN, and HFG, ensuring merger of end-user operational needs with applicable healthcare codes and guidelines.
This process involves optimizing the later hospital design development, balancing regulatory requirements with flexibility for future adaptability and facility growth. By engaging key stakeholders and analyzing space requirements, we create comprehensive design guideline document will cover the following key elements of the hospital planning process:
| Section | Description |
| Project Vision & Goals | Mission, service philosophy, population served |
| Clinical Service Scope | List of medical services (e.g., ER, ICU, OR, Imaging, Labs, etc.) |
| Workflow & Operational Model | How patients, staff, equipment, and supplies flow throughout the facility |
| Departmental Functions | Functions, adjacencies, and special needs for each department |
| Space Requirements | Number, type, and size of rooms; grossing factors; growth allowances |
| Infection Control & Safety | Zoning, separation, clean/dirty workflows, emergency exits |
| Technology & Equipment Needs | Key medical and IT equipment per service line |
| Code and Regulatory Compliance | Reference to applicable health codes, standards (e.g., FGI, JCI, MoH) |
| Sustainability & Flexibility | Energy targets, future expansion, modular design |
The deliverables of this phase is a narrative document that will constitute the basis of all the subsequent design effort.
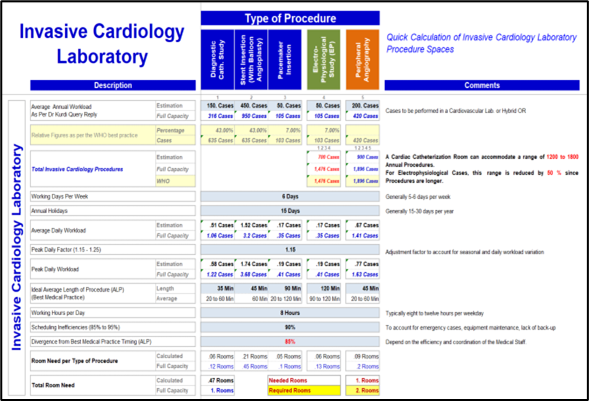
Workload and space calculations
Workload calculation and peak period strategy are crucial factors in planning hospital operations for peak workload and patient inrush handling. To accurately calculate workloads and contingency needs, we consider factors such as the number of patients, the severity of their conditions, and the required staffing levels. It is also important to take into account peak periods, such as certain times of the day or certain months of the year when patient demand is highest.

By proactively planning for peak workload periods, the planning team can ensure upfront that the designed facility can cope with the estimated workload for each department and medical activity in place, thus reducing congestion, operational bottlenecks, and patient waiting time.
The workload calculation validation will result in the procedural and treatment spaces needs such as number of operating theaters, ICU beds, Inpatient bed per specialty, emergency, procedural and treatment spaces…
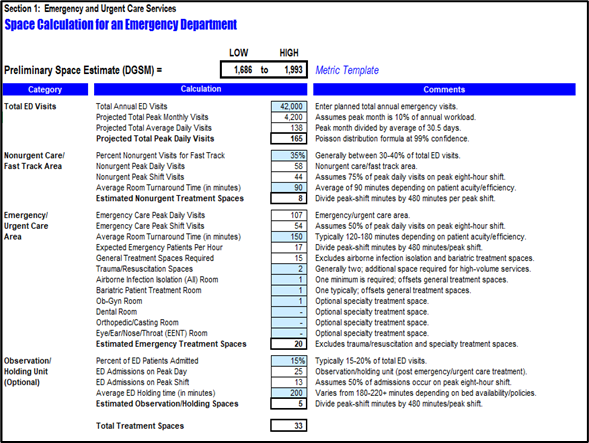
The deliverable of this stage is the Workload & Space Calculation Report.

Space program
The FSP is a multi dimensional planning report which encompasses several activities; it is the cornerstone of the planning phase.

The first component is the space program or the rooms’ schedule: a detailed list of all hospital rooms and functional areas available in all medical, ancillary, administrative and support departments along with their Net Area (NA) optimal allocation as per local and/or international healthcare guidelines and operational requirements to account for all rooms occupants spatial needs including staff, patient, visitors, equipment, furniture, circulation, clearances and accessibility to fit its intended purpose.
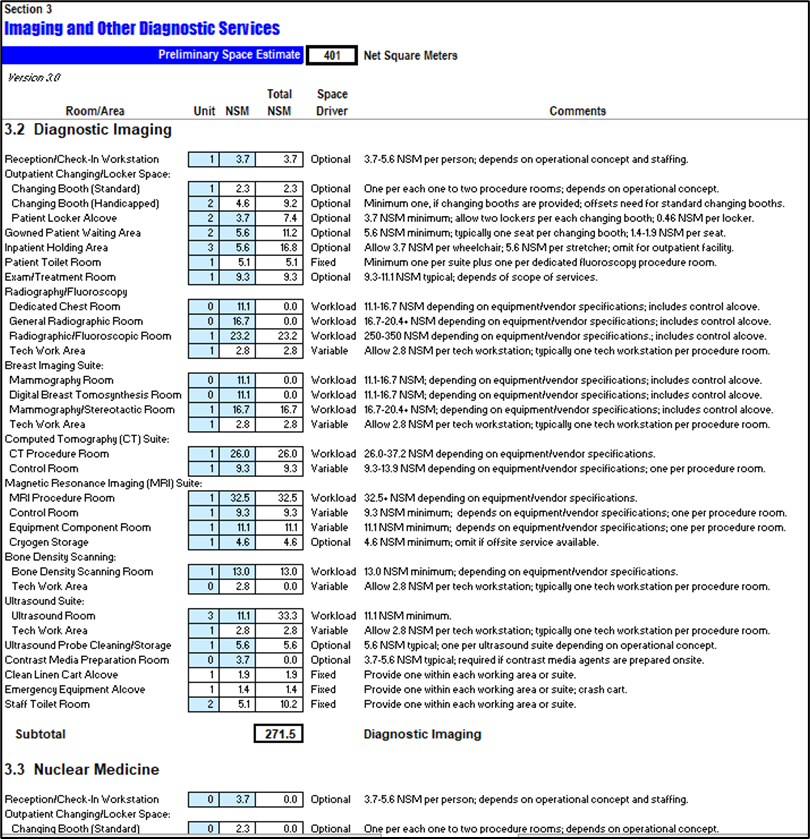
The Net Areas schedule is than converted twice into the Departmental Gross Square Area (DGSA) and finally to the Building Gross Square Area (GBSA) to account for:
- Circulation needs both horizontal and vertical
- Intradepartmental zoning
- Vertical spatial requirements
- Managed workflows and segregation policies
- Infection control, Safety

Another component of the FSP is the inter-department proximity matrix which defines the adjacency and stacking needs between departments either horizontally and vertically.
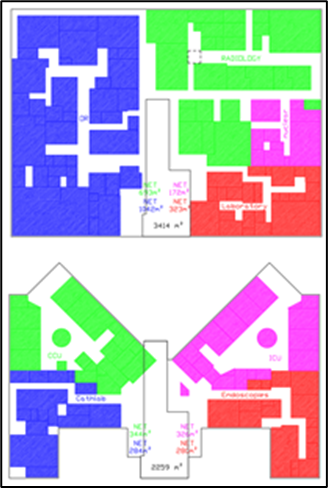
The final component of the FSP are the inter and intra- departmental Design assistance bubble diagrams to help the Architect and design team visualize the different departments’ zones circulation and adjacency needs.

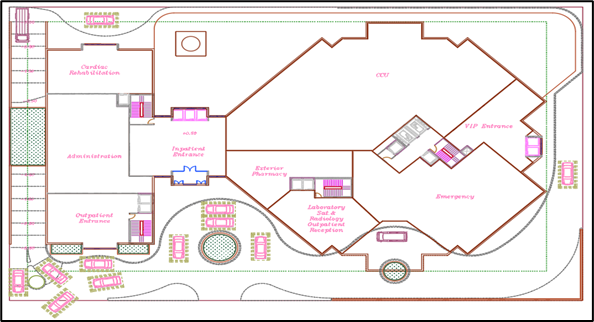
The deliverable of this stage is the Space Program which encompasses the room’s schedule, the proximity matrix and design diagrams

Medical equipment concept report
Our Medical Equipment Concept Report is developed to align the client medical equipment selection with both the latest development in medical technology and international regulatory bodies requirements such as the WHO guidelines, ensuring that all medical equipment is selected based on operational needs, safety, efficiency, and operational costs control.
This process involves engaging key stakeholders to identify equipment requirements, evaluating options against criteria like functionality and cost, and integrating equipment into workflows to enhance patient care.

Our generated Medical Equipment Concept Report aligns equipment features and options with facility requirements and patient casemix, prioritizing right-sizing to optimize efficiency and minimize total cost of ownership (CAPEX and OPEX), while ensuring regulatory compliance and patient safety throughout the equipment lifecycle.
It constitutes a comprehensive benchmark that is tailored to the on-hand medical facility en-users unique needs, supports both current and future healthcare demands, and ensures that the facility is equipped to deliver the adequate quality of care in a safe and cost-effective environment.
The Medical Equipment Concept Report is a narrative document that will constitute the basis of the subsequent equipment tendering and procurement activities.

Medical IT strategy
Information technology in healthcare has become an essential component of the medical services delivery. As technology continues to evolve into an increasingly digital world, traditional ways of delivering health care have become increasingly outdated. Hospitals need to be at the forefront of new IT technologies to provide patients with safer, faster, integrated and efficient services while lowering their costs.
PRIME can develop the medical IT strategy for its clients that covers Hospital Information System (HIS) & Enterprise Resources Planning (ERP) definition and specifications development, including systems and integration needs of the different medical technology platforms and components into the HIS such as the radiology RIS and PACS, laboratory LIS, pharmacy (PhIS) and blood bank (BBIS) information systems, ICU and OR equipment, diagnostic modalities integration requirements (EEG, ECG, PFT, EMG…), patient portal interfacing requirements, medical systems (Master clock, Nurse-call NCS, real-time location (RTLS), paging and codes systems)…

PRIME will develop a medical IT strategy that will enhance daily operations management and optimize both the hospital infrastructure and technology utilisation to:
- Manage hospital beds efficiently and maximize utilization of resources
- Streamline performance and automation of patient care processes and clinical decision support at point of care
- Optimize gathering, storing and retrieval of relevant demographic and clinical information
- Maintain a permanent record over the Electronic Medical Record and document all medical and legal requirements
The deliverable of this study is the Information Technology IT Strategy Report

Waste management strategy
Medical waste management and logistics are a critical issue in the healthcare industry operations. Hospital generated waste are a special type of waste which carries higher potential of infection and staff occupational hazards.
Hospitals and healthcare facilities generate different types of waste which span from regular waste to infectious waste, pathological, pharmaceutical, sharps, chemicals, cytotoxic and radioactive wastes depending on the type of medical activities and procedures being performed.

Proper waste management has 2 main drivers:
§Infrastructure drivers where the health care facility infrastructure design is key for proper physical segregation from the waste generation sites, temporary storage, segregated and safe routing to central separate staging areas and finally treatment whether onsite by any technological means such as autoclaving, microwaving, steam treatment integrated with internal mixing, and chemical treatment or offsite through any third party.
§Operational drivers which include key elements in improving health-care waste management such as (1) promoting practices that reduce the volume of wastes generated (2) improve operational waste segregation and disposal practices (3) building a comprehensive system through effective policies and procedures implementation (4) raising awareness through proper training to protect people from hazards when collecting, handling, storing, transporting, treating or disposing of waste.
PRIME experts will target both drivers to institute a comprehensive waste management strategy for efficient management and handling in the healthcare setup to ensure high levels of patient safety and positive clinical outcomes, low servicing costs, peak efficiency, removal of logjams and maximizing sustainability performance.
The deliverable of this study is the Waste Management Strategy Report

Infection control strategy
Healthcare-associated infections (HAIs) can produce serious aggravation in patient complication that may lead to death and are one of the leading causes of medico-legal cases; yet, half of all HAIs are preventable. Worldwide, the annual number of HAIs climbs to hundreds of millions of patients. HAIs are now the most frequent adverse events in healthcare delivery, costing an estimated $9.8 billion annually, leaving lives, your organization’s bottom line, and its reputation, at risk.

Infection control starts from the early design stages were many factors are considered such as room design, flow patterns, medical device reprocessing, disinfection and sterilization processes, and safe sterile storage. It also defines important electromechanical systems setups such as water treatment, air quality, air change and pressure gradient in the HVAC system. PRIME team experts with extensive education and experience in Infection Control in acute, ambulatory, long-term care and health & Safety. PRIME offers our clients individualized packages to suit their needs, offering independent infection control reviews, policies & procedures, blueprint/drawing reviews. This is significant in surgical theatres, CSSD, endoscope reprocessing, dirty and clean utilities, and special classified rooms (clean surgical rooms, isolated ICUs, and dialysis rooms).
PRIME ‘s consulting team helps the institute to respond to a multitude of issues related to infection control and prevention and proactively take action to mitigate infection risk. Our certified experts conduct on-site consultations. Our multidisciplinary team reviews findings and provide additional recommendations. Our team combined years of experience and expertise serve to help your facilities benefit from current best practices in healthcare.
The deliverable of this study is the Infection Control Audit and Strategy Report

Conceptual and schematic design
Conceptual design represents the start of the design process. Conceptual drawings are preliminary sketches and illustrations that depict the initial design ideas and concepts. They are used to communicate and refine ideas with the project stakeholders – specifically the Client and his Team. Conceptual drawings establish the basic form, layout, and functionality of a the proposed design before moving on to more detailed plans and specifications
Conceptual drawings are developed as an agreed upon representation to visualize the departments space allocation, zoning, proximity, equipment installation and delivery routing, inter- and intradepartmental relationships in addition to horizontal and vertical accessibility in order to properly segregate the different circulation flows patterns of patients, staff, sterile & clean materials, waste and outpatients.
Once approved by the client, the conceptual drawings are further developed and detailed into schematic drawings. The internal spatial configuration of each department starts to appear based on the selected design criteria. Preliminary coordination between the different engineering trades starts at the schematic design in order to accommodate for all technical dependency constraints early on in the design process.

The deliverable of this stage are Concept and Schematic Design Drawings

Loaded drawings with MEP requirements
LOADED DRAWINGS with MEP REQUIREMENTS – ME&F BOQ – Room Loading List (RLL) Report – Equipment Distribution Report :
The loaded drawings are the ultimate representation of the populated different rooms and areas in an effort to ensure that the allocated special areas are fit for their intended purpose and that all clearances and accessibility requirements are provided.

The hospital plans are loaded with the medical equipment and hospital furniture to the proper scale; detailed electromechanical inputs are also loaded to provide the engineering designers with the equipment’s MEP requirements to show the distribution and location of major electro-mechanical loads, including water supply, drainage, exhaust and gas outlets, main power supply, emergency power supply, uninterrupted power supply (UPS), medical gas, general lighting intensities, emergency lighting conditions, and any other special electro-mechanical requirements related to hospital-specific conditions.

The Loaded Drawings serve as a clear manifestation of the architectural design, especially as it relates to flow patterns and equipment placement requirements.
The loaded drawings are essential to generate the actual medical equipment and clinical furniture (ME&F) count and bills of quantities (BOQ). Loaded Layouts are developed on a scale of 1:100
The deliverables of this phase are: 1:100 Loaded Drawings – Medical Equipment & Furniture Bills of Quantities (ME&F BOQ) – Room Loading List (RLL) Report – Equipment Distribution Report

Room data sheets
During the design development stage, the Room Datasheets (RDS) are considered the ultimate communication tool between the planner and both all engineering trades and the Client. They represent a detailed description of each type of room specifications, architectural and MEP requirements in addition to a listing of all its equipment, furniture and fixtures. The following are part of the technical information provided by the RDS: Briefed Area – Hours of Operation – Type an number of Occupants ( patient, staff, visitors) – Acoustic privacy – HVAC Requirements – Lighting – Medical Gas – Nurse Call – Other MEP Systems – Shielding – Special Static Loads – Finishing and Fabric Requirements (Floor, Walls, Ceiling) – Room Medical Equipment & Furniture – Other Special Requirements.

The RDS are complemented by additional sets of 1:50 or 1:20 loaded drawings that are developed for some specific medical locations for additional details incorporating descriptive and quantified coded lists of equipment placed in each medical or paramedical space.
The deliverables of this phase are the: Room Datasheets Document(RDS) – 1:50 and 1:20 Detailed Loaded Drawings

Medical equipment specifications and Budgeting
Medical specifications and tender book are prepared for all medical equipment and clinical furniture (ME&F) within the scope of the project ME&F BOQ and Budgets. PRIME ensures that all medical equipment specifications are accurately developed in compliance with international standards, clinical requirements, and hospital infrastructure constraints. Prime experts ensure that specific suppliers’ neutral technical performance specifications checklists are prepared for each piece of medical equipment and clinical furniture to include the following sections:
- Scope of equipment and project
- Technical mandatory specs and optional features
- Standards, Codes and Specifications Compliance with standards (FDA and/or CE)
- Warrantees
- Systems operating costs and total cost of ownership
- Testing and Inspection
- Documentation, historical hazard alerts and product recalls
- Confirmation that ME&F are current and no end of production / support service / life are scheduled
- Software/ system interface
- Consumables and disposables
- Accessories
- Addition options and systems upgradability
- Site preparation & installation requirements
The deliverable of this stage are the ME&F Book of Specifications and ME&F Budget

Mechanical electrical plumbing
Mechanical electrical plumbing (mep) design services:
PRIME has an extensive experience in medical MEP design services that takes into consideration the specific requirements of the medical field based on the highest applicable international standards to promote safe working environment in terms of: back up and redundancy systems availability – infection control – Air quality – pressure differentials – Airborne infection and protective isolation rooms – Laminar flow for special theaters and Iso classified clean workrooms – Liquid waste water segregation and treatment – Building Management System – Automation and Patient controls

Mechanical Trade Works:
- HVAC System: Chilled Water – VRV – Heating – Ventilation – Air Conditioning
- Plumbing : Domestic Cold & Hot Water System – Water Treatment System
- Drainage and Liquid waste Treatment System (radioactive, hazardous waste)
- Fire Fighting System
- Central Technical Steam System
- Medical Gases System
- Fuel and LPG Systems
- Building Management System
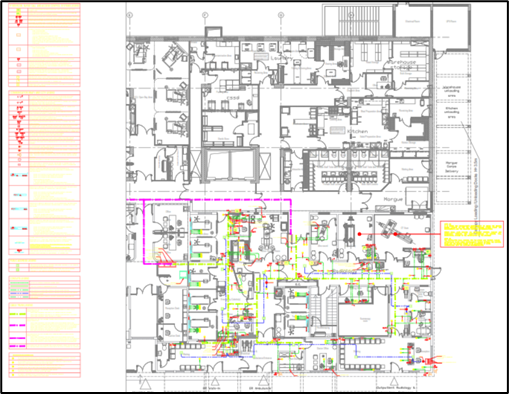
Electrical Trade Works:
- Lighting (general, task, and emergency systems)
- Power distribution systems including Normal & essential network – Isolation transformer network emergency
- Back up generator system
- Uninterrupted power supply (UPS) system and automatic transfer panels
- Nurse call system and Realtime location system / Baby abduction system (RTLS)
- Telephone & public address system (paging and music systems)
- Security surveillance and CCTV
- IT systems
- Fire alarm system
- Building Management System


The deliverable of this stage are the MEP Design Development Drawings, the MEP Construction Documents along with Budgeted BOQ

Medical gases design review services
Medical gases are classified medical drugs as per international US and European Pharmacopeias that develop and enforce medication quality and compliance.
Medical gas pipeline systems (MGPS) are installed to provide a safe, convenient and cost-effective central storage and delivery system all to the clinical and nursing point-of-use.

PRIME offers full medical gas systems planning, design review and installation works supervision. These services are based in accordance with the latest British Standards and Health Technical Memorandum; HTM 02-01.

PRIME also offers comprehensive Piped Medical Gases Compliance Testing and Verification Services of medical gas systems as per HTM 02-01.
The deliverable of this stage are the Medical gas Design Development Drawings, Construction Documents along with Budgeted BOQ

Interior design & finishing material specifications
Research and activity based design has confirmed the effect of Hospital interior Design on the patients’ psychological state, well being and overall experience rating of the hospital stay.
Interior design is crucial to:
- Reduce the patients’ stress and discomfort
- Promote a non-institutional healing environment image for patient well-being and faster recovery
- Enhance personnel efficiency and reduce operational and medical errors
- Promote infection control and biological growth inhibition
- Enhance finishing material resistance to harsh repetitive cleaning chemicals and procedures
- Optimize safety with a more reliable user traffic organization
- Promote a medical professional image of the facility
PRIME experts perform interior material selection and/or full interior design for the developed design (DD) floor plans based on international standards and the client vision & need to achieve a comprehensive interior design package that promotes a non-institutional healing environments along with proper durable material selection.
The systematic steps taken to reach fully developed interior design are coordinated throughout all the design process, to ensure that all interior design requirements are fully met. 3-D images are established to show the end-users the final anticipated product.
The deliverable of this stage are the interior design layouts including floor plans, perspectives and 3D renders

Radiation shielding design
Some medical equipment and medical procedures may generate X-rays or gamma rays that are similar in nature but differs in origin where X-ray are electronically generated radiation will gamma rays are emitted due radioactive isotopes decay. Radioactive shielding is necessary to ensure that all exposure levels to the public and medical personnel are maintained below the regulatory limits. PRIME provides:
- Architectural drawings of equipment layout
- Architectural drawings of surrounding areas indicating usage of these areas – offices, restrooms, corridor, exterior, etc.
- Elevation view of room or construction of floor and ceiling and distance between floors
- Shielding calculation for all radiology areas ( PET,CT, MRI, X-ray, nuclear medicine) , which complies with international guidelines and regulatory, including dimensions and type of concrete or lead shielding

The deliverables of this study is the Radioactive Shielding Design Calculation and Drawings

Classified clean rooms
Clean rooms are spaces in which the concentration and type of airborne particles is controlled and constructed with special selection material with low emissions of particles such as volatile organic compounds (VOCs). Clean rooms are to be used with strict operational policies in a manner to minimize the introduction, generation, and retention of particles inside the room and where other relevant parameters, e.g. temperature, humidity, and pressure, are controlled as necessary.
Clean rooms are used in different industries including pharmaceutical production, healthcare, electronics and aero-spatial development.
Clean rooms and special controlled environment units need is on the rise in the healthcare industry. In the Hospital setup, current standards require implementation of clean rooms in some operating theater setups (ISO 8 or higher), pharmaceutical and radiopharmaceutical compounding in addition to GMP bone marrow production and transplantation.
PRIME partners with key leaders in the field to provide both classical and modular facilities design and planning, validation of rooms, auditing, quality assurance, and developing SOPs for all work inside these specially classified areas to meet and maintain in the daily operation utilization of those facilities all applicable regulations including FDA, GMP, ISO, EMEA and USP based on the client or industry needs.
The deliverables of this stage are the Clean Rooms Design Development Drawings, Construction Documents along with Budgeted BOQ

Signage study and design
Signage and wayfinding are important in a hospital premises to direct patients staff and visitors through the different areas to regulate access to semi and restricted areas. Signage reflects how a facility interact with its users through its built environment and constitute one of the main drivers for a cognitive perception of the facility branding, image and positioning.

Signage and wayfinding systems should comply with local authority guidelines. PRIME provides practical and concise wayfinding solutions design and strategy.
PRIME experts will guide, advice and assist the Client along the whole signage development process for their facility. Getting to know the Client vision and needs is the key element to successfully reveal the ideal signage solutions for their projects. PRIME experts will conceptualize the general scope of the project and work closely with client on reviewing site plans and designs, communicate brand identity, address budgeting and resolve any complex tasks and functionality issues. PRIME gets all the facts and figures, so that the signage will fit the environment, client’s needs and assuring that PRIME provide a bespoken sign solution.

The deliverable of this stage are the Signage Report, Schedule and Drawings

Food and catering services design
The Catering Unit provides food services for all patients categories including inpatients, outpatients, ambulatory Patients in addition to staff and sometimes visitors. The food services may also include catering for meetings and functions, such as board meetings, seminars, conferences and special occasions. Catering Units, in healthcare facilities, deliver food to a highly susceptible population who may experience foodborne diseases as they may be immunocompromised, sick, frail or newborns.

As a result, catering in the medical setup is challenging due to the diverse dietary needs of the population served based on their medical condition. One crucial element is to provide adequate hot and cold supply chains in order to limit food contamination and bacterial growth.
Depending on the selected food delivery model whether Cook & Serve, Cook & Chill to later Reheat or Offsite Pre-prepared Meals Delivery and Bedside (Floor) Rethermalizing, PRIME offers a full catering services design study from space programming & conceptual design, capacity and peak demand determination, logistical supply planning, finished product flow & distribution to design development, equipment definition, and MEP complete comprehensive.
The deliverables of this activity are the food & catering services full Architectural and MEP Design Development Drawings, Construction Documents, Equipment Definition & Specs along with Budgeted BOQ
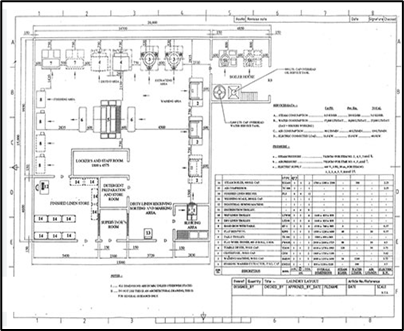
Laundry services Design
Laundry services, whether onsite, offsite or subcontracted to an external service provider, are an essential component of a proper healthcare delivery system where workflow segregation is once again key for proper handling, infection control and staff occupational hazards safety.
Hospital laundry department receives sorted soiled linen from different areas like inpatient unit, Operating Theater, OPD, ICU where they undergo several stages like sluicing, washing, drying, inspection, ironing, folding and storage or delivery. The soiled clinical linen (infected) needs special care since it has to be decontaminated & washed carefully.

Laundry services sizing and throughput capacity calculation depends on several factors such as the number of patients and surgical procedures performed, the extent of utilization of single use items, linen inventory size, working hours and shifts… Also, it differs between simple staging and storage unit with proximity to loading / unloading docks for offsite laundry services subcontracting and a full fledge laundry services in the case of onsite laundry reprocessing.
Depending on the strategic decision whether to launder onsite or contract with an external service provider, PRIME offers a laundry services full design study from workload calculation, space programming & conceptual design, capacity and peak demand determination, logistical supply planning, finished product flow & distribution to design development, equipment definition, and MEP complete comprehensive.
The deliverables of this activity are the Laundry services full Architectural and MEP Design Development Drawings, Construction Documents, Equipment Definition & Specs along with Budgeted BOQ

Tendering support and submittals evaluation
PRIME SOLUTIONS provides expert biomedical engineering support during the tendering and submittal evaluation phase of healthcare facility construction projects. Acting as a key technical advisor, PRIME reviews tender documentation to confirm that equipment requirements are clearly defined and fully coordinated with architectural, mechanical, and electrical systems.
Upon receipt of vendor bids, PRIME experts perform a comprehensive evaluation of all biomedical submittals to verify compliance with the tender specifications. This includes identifying non-conformities, obsolete technologies, or unsupported features, and preparing detailed technical comparison matrices to guide transparent and justified procurement decisions. In parallel, PRIME conducts a thorough assessment of the risks, maintainability, and long-term viability of each proposed solution.
The scope of services provided by PRIME during tendering and evaluation includes:
- Verify equipment technical specifications in alignment with healthcare service needs
- Ensuring coordination of biomedical systems with IT, MEP, and infection control strategies
- Reviewing and evaluating technical submittals for compliance, completeness, and clinical appropriateness
- Preparing formal evaluation reports and technical comparison summaries to support decision-making
- Advising on lifecycle costs, operational risks, and the sustainability of the proposed technologies
- By carrying out these responsibilities, PRIME SOLUTIONS ensures that the selected medical equipment is optimized for clinical performance, operational efficiency, and long-term value throughout the facility’s lifecycle.
The deliverable of this service are the tender evaluation documents

Project procurement management
Early medical equipment and clinical furniture (ME&F) procurement planning is critical to the development of an effective and complete procurement management plan. This schedule can then be incorporated into the overall project schedule.
Medical Procurement is the process of tendering, bids evaluation, awarding and purchasing of medical equipment & clinical furniture, surgical instruments, consumables and medical supplies.
PRIME procurement management services will add the following values to the project management activities and subsequent facility overall life cycle:
- Transparent procurement process management from tendering, bids evaluation to awarding and purchasing
- Right quality equipment procurement that is specs compliant, of current technologies at negotiated competitive budget
- Coordination of equipment procurement schedule and interdependencies with the project overall schedule
- Long-lead items planning
- Coordination of major fixed equipment procurement that will require specific site preparation and MEP adaptation
- Scheduling of equipment site delivery
- Management of the equipment site delivery activities, testing &commissioning and staff
The deliverable of this study is the Medical Project Procurement Management Plan and its Implementation

Project management for biomedical Trade activities
PRIME provide project management activities for the biomedical trade from ME&F site delivery, supervision on installation until its completion, testing and commissioning, training, substantial and final ME&F handing over and contract closure; PRIME assists in all coordination works between all engineering trades for the contractor in the corresponding decision making in the execution of the work.
The documents that will serve as basis for the biomedical project management works are all the plans, technical specifications, bill of quantities, chronogram, as well as the budget for the execution of the work by the Contractor.
The scope of services provided by the PRIME will include:
- Supervise, direct, monitor and coordinate with Main Contractor, Trade-Contractors and Sub-Contractors for all engineering trades works on site
- Ensure that all works performed conforms with contract documents requirements
- Measure the quantities of work executed, in order to verify, certify and approve the work estimates
- Verify, coordinate and approve of medical trades shop drawings plans, details or adjustments within the framework of what was contracted during the execution of the work
- Perform quality assessments on all biomedical related works
- Ensure warranty and maintenance requirements compliance with tender requirement
- Manage project schedule, long lead items, planned sequence and interdependencies of works
- Assist in managing all biomedical third party contracts related to the project
- Assist in ensuring that all necessary site preparation works are completed by all engineering trades especially for equipment that will require specific site preparation and MEP adaptation
- Assist in all biomedical related works activities are completed in a timely manner to keep project schedule on target and within budget
- Recommend and advise on Change Orders for the adequate execution of the works
- Participate in the testing and commissioning, provisional reception and acceptance of the work, and prepare the biomedical corresponding report including the list of observations/ snagging to be fulfilled by the contractor
- Participate in the final reception and acceptance of the work and prepare the final supervision report, with the recommendation for the financial settlement of the contract
- Manage end users training and capacity building
- Assist in the substantial and final ME&F handing over and contract closure
The deliverable of this study is the Biomedical Project Management Plan

Testing and Commissioning
PRIME experts provides end-to-end biomedical testing and commissioning services to ensure all medical equipment is installed, configured, and functioning in accordance with project specifications and clinical requirements. PRIME supervises on-site testing procedures, coordinates with vendors and engineering teams, and ensures all systems are verified for operational readiness.
Upon completion of installation, PRIME leads the commissioning phase, which includes functionality checks, calibration, and safety testing of all biomedical systems. PRIME ensures that each item is validated against manufacturer specifications and local regulatory standards before being accepted.
In parallel, comprehensive user training is organized for clinical and technical staff to ensure safe and effective use of all equipment. PRIME prepares and manages the full handover documentation, including test reports, service manuals, warranty certificates, and maintenance plans, ensuring a seamless transition from construction to operational readiness.
The deliverable of this service are the testing and commissioning and end-user training reports

Facility evaluation/Retesting & Commissioning
As part of a comprehensive facility evaluation audit, PRIME multidisciplinary experts inspect every part of a building’s infrastructure, MEP systems and biomedical technologies to gather information regarding its condition, deficiencies and functional efficiency & effectiveness.
The main objective of the facility evaluation is to measure the condition and functionality of building and its infrastructure as suitable and appropriate for its intended functions. Specific objectives of the comprehensive assessment include determining needs for renewal or replacement of building and infrastructure systems (e.g., Heating/cooling, electrical, exterior envelope, etc.) and system components (e.g., cooling tower, heat exchanger, chiller, pumps, etc.), and guiding the analysis of good decision capital project options, including renovation or modernization.

Another type of facility audit is the gap evaluation which could be compared to an inventory assessment- a list of what a facility has and what it needs in order to function effectively and safely while it produces medical services. It is an essential tool required prior to any infrastructure or system decision whether to revamp, rehabilitate, upgrade or replace. The assessment serves as a “status snapshot” and “road map” showing what needs to be done and what are the different incurred costs (cost of investment – cost of quality / lack of quality – loss of earnings).
PRIME provide project management activities for the biomedical trade from ME&F site delivery, supervision on installation until its completion, testing and commissioning, training, substantial and final ME&F handing over and contract closure; PRIME assists in all coordination works between all engineering trades for the contractor in the corresponding decision making in the execution of the work.
The documents that will serve as basis for the biomedical project management works are all the plans, technical specifications, bill of quantities, chronogram, as well as the budget for the execution of the work by the Contractor.
The scope of services provided by the PRIME will include:
A comprehensive facility assessment has 3 major components:
- Condition Assessment
- Functional Assessment
- GAP Assessment
Our trained experts utilize walk-through inspection, research and baseline data collection as a basis. This analysis allows us to identify: architectural, structural, mechanical, electrical, plumbing, medical systems, biomedical equipment and site conditions.
Our team will utilize this analysis to determine the adequacy of a building to house its intended function. This data answers important questions, which will help with routine building facility management activities including long-range budgeting and modernization planning.
A full detailed evaluation report enables the owner and management to identify the condition of the facility, and what needs to be done to upgrade & update it, to comply with current safety and design regulations.
Once a decision is made, the full PRIME scope of work can be systematically applied for a facility life cycle revamping from rehabilitation design until commissioning and project completion.
The deliverable of this study is the Facility Evaluation Report
Facility Management Services include the following activities and services ensuring clarity in roles, responsibilities, and compliance with industry standards:
1. Policy and Compliance Development
- Policy, Procedure and Standardized Processes Development (BESA & JCI Compliant)
- Quality Control and Compliance Checks
- Assessments and Compliance Checks with Safety Regulations and Building Codes
2. Asset Management
- Asset Capturing, Registry and Unique Codification
- Non-Medical/General Equipment and Systems History Capturing and Life Cycle Management
3. Maintenance Management
- Maintenance Software Deployment, Initialization and Management
- Daily Inspection For All Infrastructure Systems and Major Operations Support Equipment
- Preventive(PM), Predictive (PdM) and Regular Inspections Based On BESA Requirements
- Corrective Maintenance (CM), Repairs and Upkeep Based on BESA Requirements
- Service Contracts Management for Specialized Equipment and Systems
4. Health and Safety Programs
- Occupational Health & Safety Program for Hospital Staff And Technical Teams (To ensure a safe working environment)
- Infection Control Protocols Implementation: HVAC/Air and Water Culture and Quality Checks
5. Procurement Support
- Support Client’s Procurement Department for all Non-Medical/General Equipment and Systems Acquisitions and Replacements
- Support in Evaluation Studies
- Support in Tendering and Evaluation Process
6. Training and Development
- Training for Hospital Staff and Technical Teams
7. Reporting and Monitoring
- Continuous Reports to Management (Daily/Weekly …)
- KPIs and Operation Monitoring
- Quality Report
- End of Year Economic Report
- Non-Medical/General Equipment Replacement Assessment Report
- Dashboard and Reporting
- Risks Assessment Report
- Facility State Evaluation Report
- Gaps Analysis Report
8. Facility and infrastructure audit:
- Conduct thorough inspections of physical assets, including HVAC systems, electrical installations, plumbing, and structural elements
- Gather data through observations, interviews with staff, and reviews of maintenance records
- Data collection and Analysis in order to improve efficiency and cost saving
9. Maintenance Department Audit:
- Analyze Past Maintenance Issues to Identify Underlying Problems and Verify The Existence of a Continuous Improvement Process
- Evaluate Overall Performance Against Predefined Objectives
- Assess The Performance and Effectiveness of The Maintenance Department’s Workforce
- Evaluate The Clarity and Effectiveness of Documented Maintenance Policies, Procedures and Department Processes
- Assess The Efficiency of Corrective Work Order, Preventive and Predictive Maintenance Management
- Conduct Thorough Inspections of Physical Assets, Including HVAC Systems, Electrical Installations, Plumbing, and Structural Elements
- Analyze Metrics such as Mean Time Between Failures (MTBF), Mean Time To Repair (MTTR), and Equipment Uptime
- Examine Historical Maintenance Records to Identify Trends and Areas for Improvement
- Confirm Adherence to Industry Regulations and Safety Standards
- Assess The Adequacy of Training Programs for Maintenance Staff, Ensuring They Align with Skill Requirements
- Conduct a GAP And SWOT Analysis for The Maintenance Department, and Develop an Action Plan/Roadmap for Departmental Restructuring
9. Energy Audit
- Identify Energy Waste
- Enhance Efficiency
- Cost Savings
- Compliance
Lines of Services: Facility Management
Facility Management Services cover the following systems and infrastructure elements:
HVAC SYSTEMS:
- Chillers and Cooling Towers
- Fresh Air Handling Units (AHU & FAHU), Fan Coil Units (FCU), Split units
- VRV/ VRF system, VAV system
- Filtration Systems
- Pumping Stations
- Ducting Networks
- HVAC Controls

ELECTRICAL SYSTEMS:
- Power distribution system, including MDBs, ATSs, and FDBs
- Standby and Emergency Generators with Synchro
- Uninterrupted Power Supply (UPS)
- Solar Systems
- Isolation Transformers
- Earthing systems
- Lighting Systems: Interior and Exterior Lighting, Emergency and Exit lighting
- Wiring for power, lighting, Telephone, Fire Alarm, Security systems.

LOW VOLTAGE SYSTEMS:
- BMS, SCADDA, IOT
- Communication Systems
- Telephony and Intercoms
- Nurse Call Systems
- Data Networks
- Security System
- Surveillance Cameras CCTV
- Alarm Systems
- Access Control System

FIRE FIGHTING:
- Fire Panels
- Smoke and Heat detectors
- Fire Pumps
- Hose reels and Cabinets
- Sprinklers
- Emergency Notification, Speaker and Addressing Devices
- Fire extinguishers
WATER & STEAM SYSTEMS:
- Water Treatment and RO Plants
- Sewage Treatment Plants
- Boilers
- Steam Generators
- Drainage and Water Networks
- Water tanks
- Water Pumps
- Sanitary Fixtures
- Rain Water Collection
EQUIPMENT & FURNITURE:
- Kitchen Equipment
- Sterilization Equipment:
- Autoclaves, Washers, and Flushers
- General Furniture:
- Medical Beds, Overbed Tables, Exam Tables
- Carts and Trolleys
- Dialysis & Infusion Chairs
- Chairs
- Fixture Elements Counters…):
- Nurse Stations
- Reception Desks
- Wardrobe Cabinets
Building Infrastructure
- Structural Systems:
- Roofs & False Ceiling
- Walls
- Flooring
- Windows & Doors
- Accessibility and Transportation:
- Elevators & Dumbwaiters
- Escalators & Ramps
Lines of Services: Biomedical/Clinical Engineering
1. Policy and Compliance Development
- Policy, Procedure and Standardized Processes Development (JCI Compliant)
- Assessments and Compliance Checks with Safety Regulations, WHO and Medical Equipment Codes
2. Asset Management
- Asset Capturing, Registry and Unique Codification
- Medical Equipment and Systems History Capturing and Life Cycle Management
3. Maintenance Management
- Maintenance Software Deployment, Initialization and Management
- Preventive(PM), Predictive (PdM) and Regular Inspections using Certified & Calibrated Analyzers
- Corrective Maintenance (CM), Repairs and Upkeep Based on Equipment Manufacturers’ Requirements and Biomedical Best Practices
- Service Contracts Management for Specialized Equipment and Systems
4. Quality Control and Calibration Checks
- Quality Control for Medical Equipment
- Calibration and Compliance Checks Based on Equipment Manufacturers’ Requirements
Biomedical/Clinical Engineering Services include the following activities and services ensuring clarity in roles, responsibilities, and compliance with industry standards:
- Policy and Compliance Development
- Policy, Procedure and Standardized Processes Development (JCI Compliant)
- Assessments and Compliance Checks with Safety Regulations, WHO and Medical Equipment Codes
- Asset Management
- Asset Capturing, Registry and Unique Codification
- Medical Equipment and Systems History Capturing and Life Cycle Management
- Maintenance Management
- Maintenance Software Deployment, Initialization and Management
- Preventive(PM), Predictive (PdM) and Regular Inspections using Certified & Calibrated Analyzers
- Corrective Maintenance (CM), Repairs and Upkeep Based on Equipment Manufacturers’ Requirements and Biomedical Best Practices
- Service Contracts Management for Specialized Equipment and Systems
- Quality Control and Calibration Checks
- Quality Control for Medical Equipment
- Calibration and Compliance Checks Based on Equipment Manufacturers’ Requirements
- Procurement Support
- Support Client’s Procurement Department for all Medical Equipment and Systems Acquisitions and Replacements
- Support in Feasibility and Evaluation Studies
- Elaborate Technical Specifications
- Support in Tendering and Evaluation Process
- Management of Procured Medical Equipment and Systems Installation, Testing and Commissioning, and Handing Over
- Training and Development
- Training for Hospital Staff and Biomedical Teams
- Reporting and Monitoring
- Continuous Reports to Management (Daily/Weekly …)
- KPIs and Operation Monitoring
- Quality Report
- End of Year Economic Report
- Equipment Replacement Assessment Report
- Dashboard and Reporting
- Risks Assessment Report
- Medical Technology and Equipment Status Evaluation Report
- Gaps Analysis Report
Biomedical/Clinical Engineering Services cover the following medical equipment and systems :
MEDICAL EQUIPMENT:
- Anesthesia Machines
- Ventilators
- Operating Tables
- Defibrillators
- Automated External Defibrillators (AEDs)
- ECG Machines
- Cardiac Monitors
- Holter Monitors
- Infusion Pumps
- Dental Chairs
- Dialysis Machines
- Microscopes
- Centrifuges
- Spectrophotometers
- Surgical Instruments
- Oxygen Cylinders and Masks
- Hand-Held Thermometers
- Blood Pressure Monitors
- Pulse Oximeters
- Glucose Meters
SPECIALIZED EQUIPMENT:
- X-ray Machines
- MRI Scanners
- Ultrasound Machines
- CT Scanners
- Radiation Therapy
- Laboratory Equipment
- Surgical Lasers
MEDICAL FURNITURE:
- Hospital Beds (Inpatient, ICU Beds, Bariatric Beds, Pediatric Beds…)
- Recliner Chairs
- Gynecological Chairs
- Examination Tables
- Stretchers
- Overbed Tables
- Delivery Tables
- Stainless Steel Trolleys
- Medical Carts (Crash/Emergency, Anesthesia…)
- Dressing Trolleys
- Wheelchairs
- Bedside Cabinets
- IV Stands
- Bedside Screens
- Crutches and Walkers
Project Management Services is handled at PRIME SOLUTIONS by a specialized Medical Projects team.
PRIME brings together a network of experts specialized in various Management, Construction, Engineering and Operations Backgrounds from facility planning, architecture, biomedical engineering to MEP engineering, hospital management and operations administrators Using advanced software tools & merging the latest international guidelines and standards with the field best practice and activity based design concepts.
